Gadolinium contrast agents have been used safely in more than 300 million MRI scans over 35 years – but knowing the real risks versus the fear-mongering helps you make informed decisions about your health.
When your doctor orders an MRI with contrast, that gadolinium-based agent makes the difference between seeing a tumor clearly or missing it entirely. The enhanced imaging reveals blood vessels, identifies infections, and characterizes masses that would otherwise remain invisible or ambiguous.
Recent concerns about gadolinium retention in the body sparked legitimate questions. The FDA issued warnings. Media coverage amplified fears. Patients started refusing necessary scans. Here’s what the actual evidence shows – gadolinium remains extraordinarily safe for most people, with specific precautions needed for a small subset of patients.
At Craft Body Scan, we balance the proven diagnostic value of advanced imaging against genuine safety considerations. Our board-certified radiologists understand when gadolinium provides essential information and when alternative approaches deliver adequate answers. Understanding gadolinium safety starts with facts, not fear.
What Gadolinium Actually Does in Your Body
Gadolinium is a rare earth metal with powerful magnetic properties. Free gadolinium is toxic – your body can’t process or eliminate the elemental form safely. That’s why pharmaceutical manufacturers create gadolinium-based contrast agents (GBCAs) by binding gadolinium to organic molecules called chelates.
Think of the chelate as a protective cage around the gadolinium ion. This cage keeps the toxic metal sequestered while allowing it to perform its imaging function. The chelated gadolinium shortens the relaxation time of nearby hydrogen protons in water molecules, creating brighter signals on T1-weighted MRI images.
This brightness enhancement transforms how radiologists visualize internal structures. Blood vessels that appear as vague shadows on non-contrast MRI become clearly defined pathways. Tumors that blend into surrounding tissue suddenly show distinct borders. Inflamed tissues light up differently than healthy tissue.
Your kidneys eliminate most GBCAs within 24 hours through urine. The chelate structure remains intact during this elimination process – the gadolinium stays bound to its protective cage and exits your body as a complete unit. In people with normal kidney function, this clearance happens efficiently.
Nine different GBCAs are currently available, divided into two structural categories. Linear agents use open-chain molecules to bind gadolinium. Macrocyclic agents employ ring-shaped molecules that wrap more tightly around the gadolinium ion. This structural difference affects stability – how strongly the gadolinium stays bound to its chelate.
The standard dose is 0.1 millimoles per kilogram of body weight. For an average adult, this translates to about 10-15 milliliters of contrast agent injected intravenously. Some protocols use half this dose with newer high-relaxivity agents that produce equivalent enhancement with less gadolinium.
The Real Safety Profile: What Evidence Actually Shows
Adverse reactions to gadolinium contrast occur less frequently than reactions to iodinated CT contrast. The overall incidence of any adverse reaction sits below 1%. Most reactions manifest as mild symptoms – nausea, headache, or injection site discomfort – that resolve without treatment.
Allergic-type reactions happen in approximately 0.07-0.7% of GBCA administrations. These range from mild itching or hives to moderate symptoms like wheezing or low blood pressure. Severe anaphylactic reactions occur extremely rarely – roughly 1 in 100,000 to 1 in 400,000 administrations.
Facilities performing contrast-enhanced MRI maintain emergency protocols and medications. Radiologists and technologists receive training in recognizing and managing adverse reactions. Patients with previous reactions to gadolinium receive premedication with steroids and antihistamines, reducing subsequent reaction rates by 80-90%.
The contrast agent itself doesn’t increase radiation exposure – MRI uses magnetic fields and radio waves, not ionizing radiation. This distinguishes gadolinium-enhanced MRI from iodine-enhanced CT scans, which do involve X-ray radiation.
Gadolinium crosses the placenta during pregnancy. While animal studies show no fetal harm, human data remains limited. Professional societies recommend avoiding gadolinium in pregnant patients unless the diagnostic information is absolutely essential and cannot be obtained through non-contrast imaging.
Breastfeeding mothers can continue nursing after gadolinium administration. Less than 1% of the administered dose passes into breast milk, and less than 1% of that gets absorbed by the infant’s digestive system. The American College of Radiology states that interrupting breastfeeding is unnecessary.
Nephrogenic Systemic Fibrosis: The Most Serious Risk
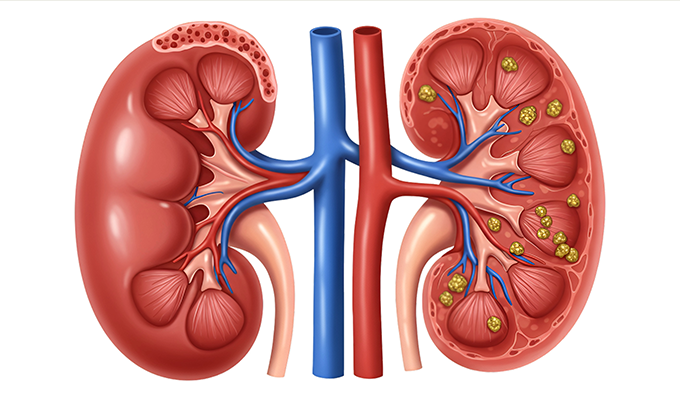
Nephrogenic systemic fibrosis (NSF) represents the most devastating complication associated with gadolinium exposure. This progressive fibrosing disease affects skin and internal organs, causing thickening and hardening of tissues that can lead to severe disability or death.
NSF was first identified in 1997, described as an independent disease in 2000, and linked to gadolinium in 2006. The connection changed medical practice dramatically. Between 2006 and 2009, hundreds of NSF cases were reported globally, primarily in patients with severe kidney disease who received certain gadolinium agents.
The risk concentrates almost exclusively in patients with advanced chronic kidney disease (estimated glomerular filtration rate below 30 mL/min/1.73m²) or acute kidney injury. Patients on dialysis face the highest risk. The mechanism involves prolonged gadolinium retention in people whose kidneys can’t efficiently eliminate the contrast agent.
When gadolinium remains in the body for extended periods, the chelate structure can break down through a process called transmetallation. Other metal ions (zinc, copper, calcium) displace the gadolinium from its protective cage. Free gadolinium then deposits in tissues, triggering inflammation and excessive collagen production.
NSF typically develops within 2-10 weeks after gadolinium exposure, though cases have occurred from days to years later. Early symptoms include skin thickening, hardening, and tightening – primarily affecting the arms, legs, and trunk. The skin may develop a woody texture with darkened patches. Progressive cases involve muscles, joints, and internal organs.
Treatment options for NSF remain limited and disappointing. Some patients showed improvement with immunosuppressive therapies, kidney transplantation (which restores gadolinium clearance), or experimental treatments. Prevention through avoiding high-risk gadolinium agents in susceptible patients became the primary strategy.
The good news – NSF cases have dropped dramatically since 2008. Regulatory changes, clinical practice guidelines, and discontinuation of high-risk agents reduced new NSF cases to nearly zero. Current guidelines classify gadolinium agents into three groups based on NSF risk, with Group II (macrocyclic) agents showing sufficiently low risk that screening requirements have been relaxed.
Modern macrocyclic agents produced zero confirmed NSF cases in multiple large studies involving thousands of patients with severe kidney disease. The 2021 American College of Radiology guidelines state that the potential harms of delaying or withholding Group II GBCAs may outweigh the minimal NSF risk in most clinical situations, even in patients with advanced kidney disease.
Gadolinium Retention: What We Know and Don’t Know
Starting in 2013, researchers discovered something unexpected. Patients who received multiple gadolinium-enhanced MRI scans showed signal changes in certain brain structures on subsequent non-contrast scans. These signal changes indicated gadolinium deposition in the dentate nucleus, globus pallidus, and other deep brain structures.
This discovery sparked immediate concern. Gadolinium was accumulating in the brain, persisting for months or years after exposure. Autopsy studies confirmed the presence of gadolinium in brain tissue, bones, and other organs of patients who received GBCAs – even those with normal kidney function.
The critical question remained – does this retention cause harm? After extensive investigation, no clear evidence links gadolinium retention to adverse clinical effects in patients with normal kidney function. The FDA concluded in 2017 that “the benefit of all approved GBCAs continues to outweigh any potential risks.”
However, the FDA required manufacturers to add warnings about gadolinium retention to all GBCA labels and develop patient medication guides. They also mandated human and animal studies to further assess safety. The European Medicines Agency went further, restricting or suspending marketing authorization for several linear gadolinium agents.
Retention levels differ significantly between agent types. Linear GBCAs show substantially more brain deposition than macrocyclic agents. This reflects their lower thermodynamic stability – linear chelates more readily release gadolinium compared to the tighter ring structure of macrocyclic agents.
Some patients report a constellation of symptoms they attribute to gadolinium retention – brain fog, joint pain, skin problems, and fatigue. This condition, termed gadolinium deposition disease or gadolinium toxicity, remains controversial within the medical community. No validated diagnostic criteria exist, causation hasn’t been established, and symptom patterns vary widely.
Research continues into the long-term implications of gadolinium retention. Questions remain about whether trace amounts cause subtle effects not yet detectable, whether effects might emerge after decades of accumulation, and whether certain individuals face higher susceptibility to gadolinium-related problems.
What’s clear – modern macrocyclic agents show minimal retention, far less than linear agents. For patients requiring multiple contrast-enhanced MRI scans over time, choosing macrocyclic agents reduces total gadolinium body burden significantly.
When Doctors Skip Gadolinium: Alternative Approaches

Radiologists increasingly recognize situations where non-contrast MRI techniques provide adequate diagnostic information. These alternatives eliminate gadolinium exposure while answering specific clinical questions effectively.
Diffusion-weighted imaging (DWI) detects strokes and tumors. This technique measures water molecule movement in tissues. Restricted diffusion appears bright on DWI images – a pattern seen in acute strokes within minutes of onset and in many tumors. DWI helps characterize masses as benign versus malignant without requiring contrast enhancement.
Arterial spin labeling (ASL) visualizes blood flow naturally. ASL magnetically labels blood water molecules as they enter the imaging area, creating contrast from the patient’s own blood without injecting anything. This technique reveals cerebral blood flow patterns, identifies areas of decreased perfusion after stroke, and helps evaluate brain tumors. Scan times run longer than contrast-enhanced sequences, but ASL eliminates gadolinium exposure entirely.
Time-of-flight (TOF) MR angiography shows blood vessels. TOF exploits the difference in signal between flowing blood and stationary tissue. It works well for visualizing major cerebral arteries and detecting aneurysms or stenosis. Limitations include difficulty imaging tortuous vessels and sensitivity to patient motion. For many vascular screening applications, TOF provides sufficient information without contrast.
Magnetic resonance spectroscopy (MRS) analyzes tissue chemistry. MRS measures concentrations of specific metabolites within tissues. Brain tumors show characteristic metabolite patterns different from normal brain or inflammation. MRS helps distinguish tumor recurrence from radiation necrosis after brain tumor treatment – a critical clinical question where gadolinium enhancement sometimes misleads.
Susceptibility-weighted imaging (SWI) highlights blood products. SWI is extremely sensitive to compounds containing iron, including blood breakdown products. It detects small hemorrhages, vascular malformations, and calcifications invisible on other sequences. Traumatic brain injury evaluation and venous imaging benefit from SWI without requiring contrast.
Amide proton transfer (APT) imaging assesses cellular metabolism. This emerging technique detects mobile proteins and peptides in tissues. APT shows promise for tumor grading, distinguishing tumor progression from treatment effects, and identifying regions of active tumor within larger masses. Clinical availability remains limited but growing.
Non-contrast techniques work best for specific diagnostic questions. Multiple sclerosis surveillance provides an excellent example. Initial diagnosis often requires gadolinium to demonstrate active inflammation. However, routine follow-up scans can use non-contrast protocols to detect new lesions and monitor disease progression. Research shows that gadolinium adds minimal diagnostic value in stable MS patients undergoing routine surveillance.
Brain tumor follow-up represents another appropriate application. After establishing the diagnosis and treatment plan with contrast-enhanced imaging, many patients can transition to alternating contrast and non-contrast scans. This reduces cumulative gadolinium exposure while maintaining adequate disease monitoring.
Limitations exist. Non-contrast techniques typically take longer than contrast-enhanced scans. They’re more sensitive to patient motion, which degrades image quality. Some diagnoses still require contrast for definitive characterization – distinguishing scar tissue from tumor recurrence, evaluating complex infectious processes, or detailed pre-surgical vascular mapping.
Emerging Alternatives to Gadolinium
Research efforts focus on developing contrast agents that provide gadolinium’s benefits without its risks. Manganese-based agents lead the alternatives currently under investigation.
Manganese offers distinct advantages over gadolinium. Unlike gadolinium, manganese is an essential trace element – your body naturally requires small amounts for normal function. Existing biological pathways process and eliminate manganese. The body contains about 10-20 milligrams of manganese at any time, distributed throughout various tissues and organs.
Manganese possesses strong magnetic properties suitable for MRI contrast. When chelated properly, manganese-based agents produce T1 signal enhancement comparable to gadolinium. Early research showed manganese could highlight blood vessels, detect tumors, and enhance tissue contrast effectively.
Mn-PyC3A represents the most advanced manganese-based agent. This compound demonstrated vascular contrast enhancement equivalent to gadolinium-based agents in primate studies. Critically, Mn-PyC3A cleared the body through mixed renal and hepatobiliary pathways – meaning the liver compensates when kidney function is impaired.
In head-to-head comparisons using renally impaired rats, manganese elimination significantly exceeded gadolinium elimination seven days post-administration. This dual clearance pathway potentially eliminates the NSF risk that devastates patients with severe kidney disease who receive gadolinium.
Phase I/II human trials evaluated Mn-PyC3A safety and clearance in healthy volunteers and patients. Results showed the agent was well-tolerated with rapid elimination. PET-MRI imaging confirmed the hepatobiliary clearance pathway functions even when kidney function is compromised. These early clinical results represent important steps toward eventual approval.
Challenges remain. Manganese itself can be toxic at high doses, particularly with chronic exposure. Free manganese ions affect the nervous system. While chelated manganese agents show low acute toxicity in preclinical studies, comprehensive long-term safety data needs development before widespread clinical use.
The contrast effect of manganese differs slightly from gadolinium due to different magnetic properties. Radiologists and technologists would need to adjust imaging protocols and interpretation approaches. The transition won’t happen overnight even if manganese agents gain approval.
Iron-based agents represent another alternative path. Some iron nanoparticles produce T1 enhancement similar to gadolinium while others create T2 effects. Iron nanoparticles can be engineered to target specific tissues or remain in blood vessels longer than standard contrast agents. Like manganese, iron is naturally processed by the body, though concerns about iron deposition exist.
Next-generation gadolinium agents with higher relaxivity allow reduced doses. Gadopiclenol, approved in 2022, produces equivalent enhancement at half the gadolinium dose compared to standard agents. Its macrocyclic structure provides high stability while the high relaxivity enables dose reduction. This approach doesn’t eliminate gadolinium but substantially reduces total body exposure.
Artificial intelligence may ultimately reduce contrast needs. Deep learning algorithms can enhance image quality from low-dose or non-contrast scans, potentially extracting diagnostic information that currently requires full-dose gadolinium. This technology remains under development but shows promise for reducing overall contrast dependence.
Making Informed Decisions About Gadolinium Contrast

Your doctor orders gadolinium-enhanced MRI when the diagnostic information outweighs potential risks. This risk-benefit calculation differs substantially between clinical scenarios.
High-value situations where gadolinium provides essential information – staging newly diagnosed cancer, evaluating stroke beyond the acute phase, characterizing complex infections, pre-surgical vascular mapping, and investigating unexplained neurological symptoms. For these indications, gadolinium frequently changes diagnosis or treatment decisions. The benefit clearly exceeds minimal risk.
Intermediate situations where alternatives might suffice – routine MS surveillance in stable patients, follow-up imaging for known benign lesions, and screening for certain conditions. Discuss with your doctor whether non-contrast techniques could answer the clinical question adequately. Sometimes they can, sometimes they can’t.
Situations where gadolinium should be avoided – severe kidney disease (unless diagnostic information is critical and unavailable otherwise), documented severe allergic reaction to gadolinium, and pregnancy (except when absolutely essential). History of multiple prior gadolinium exposures warrants discussion about using macrocyclic agents to minimize additional retention.
Questions to ask your doctor. “Is the contrast essential for this particular scan, or would a non-contrast MRI provide adequate information?” “Which type of gadolinium agent will be used?” “Given my kidney function, what are my specific risks?” “Are there alternative imaging approaches that could answer the clinical question?”
Most patients with normal kidney function face minimal risk from gadolinium-enhanced MRI. The proven diagnostic value – detecting cancers early, identifying vascular problems before they rupture, characterizing infections accurately – far outweighs theoretical concerns about trace retention. Refusing necessary contrast-enhanced imaging can delay diagnosis and worsen outcomes.
For patients requiring frequent MRI surveillance, strategies minimize cumulative exposure. Alternate between contrast and non-contrast scans when feasible. Request macrocyclic agents with lower retention. Ask about half-dose protocols using high-relaxivity agents. These approaches balance monitoring needs against long-term exposure concerns.
The Future of Contrast-Enhanced Imaging
Medical imaging continues evolving toward safer alternatives and more judicious gadolinium use. The days of routine, unquestioning gadolinium administration for every MRI are ending. Instead, radiologists carefully evaluate whether contrast provides essential diagnostic information for each specific clinical question.
Technological advances enable lower gadolinium doses while maintaining image quality. High-field strength magnets (3 Tesla rather than 1.5 Tesla) produce stronger signals, reducing contrast requirements. Optimized pulse sequences extract more information from less contrast. Artificial intelligence algorithms enhance images acquired with reduced contrast doses.
These refinements particularly benefit patients requiring lifetime imaging surveillance – cancer survivors, people with genetic conditions predisposing to tumors, patients with chronic inflammatory diseases. Reducing per-scan gadolinium exposure by 50% translates to substantially less cumulative retention over decades of monitoring.
The research pipeline includes promising alternatives. Manganese-based agents may reach clinical availability within 5-10 years if ongoing trials demonstrate safety and efficacy. Other novel contrast mechanisms continue development. The goal – maintaining or improving diagnostic capability while eliminating or minimizing metal retention concerns.
Professional guidelines increasingly emphasize selective gadolinium use. The International Society for Magnetic Resonance in Medicine advocates cautious GBCA administration only when necessary. The European Society of Urogenital Radiology developed decision algorithms for when gadolinium adds value versus when non-contrast techniques suffice.
This represents progress, not panic. Gadolinium-enhanced MRI transformed medical diagnosis and treatment over 35 years. It saves lives by detecting cancers early, preventing strokes through vascular imaging, and guiding surgical planning. The technology remains extraordinarily safe for most patients, with specific precautions for high-risk groups.
What This Means for Your Health
Understanding gadolinium safety empowers informed decisions rather than fear-based refusal of necessary imaging. The actual risks concentrate in specific patient populations – primarily those with severe kidney disease. For people with normal kidney function, gadolinium-enhanced MRI carries minimal risk while providing substantial diagnostic benefit.
The evidence supporting gadolinium safety spans decades and hundreds of millions of administrations. Serious adverse events remain rare. NSF cases dropped to near zero following the adoption of safer agents and appropriate patient selection. Gadolinium retention in brain tissue hasn’t been linked to clinical symptoms or cognitive effects in patients with normal kidney function.
Refusing contrast when your physician recommends it can delay diagnosis and potentially worsen outcomes. Early cancer detection saves lives. Identifying aneurysms before rupture prevents deaths. Characterizing infections accurately guides treatment. These benefits typically outweigh theoretical long-term concerns about minimal gadolinium retention.
That said, questioning routine contrast use makes sense. If you’ve had multiple gadolinium-enhanced scans, ask whether each subsequent scan truly requires contrast or whether non-contrast imaging could suffice. Request macrocyclic agents when contrast is necessary. These simple steps minimize exposure while maintaining diagnostic quality.
At Craft Body Scan, our comprehensive imaging services focus on early detection using optimized protocols. While we primarily perform CT-based screening, we understand the broader imaging landscape and help patients navigate questions about contrast safety across all modalities. Our board-certified radiologists stay current on evolving safety guidelines and emerging alternatives.
Our Full Body Scan at $2,495 provides thorough evaluation of your heart, lungs, abdomen, and pelvis using low-dose CT technology. This screening identifies potential problems before symptoms develop – lung nodules, vascular disease, organ abnormalities, and more. Early detection through screening gives you the best chance for successful intervention when treatment works best.
For patients with concerns about contrast agents, CT screening offers powerful diagnostic capability without requiring intravenous contrast for many applications. Calcium scoring detects coronary artery disease without contrast. Lung cancer screening requires only non-contrast CT. Many abdominal and pelvic conditions show clearly on non-contrast imaging.
The message isn’t to fear gadolinium – it’s to use it appropriately. Trust your physicians to weigh risks and benefits for your specific situation. Ask informed questions about alternatives when multiple scans are planned. Choose facilities using modern, safer gadolinium agents. These strategies optimize diagnostic accuracy while minimizing any theoretical long-term concerns.
Medical imaging will continue advancing toward safer, more effective contrast agents. Manganese-based alternatives may soon provide gadolinium’s benefits without retention concerns. AI-enhanced imaging may reduce contrast dependence entirely. Until then, selective, careful gadolinium use balances proven benefits against minimal risks.
Your health depends on accurate diagnosis. Imaging with appropriate contrast makes that diagnosis possible. Understanding the real safety profile – not the sensationalized headlines – helps you make decisions that protect your long-term health while accessing the diagnostic information you need today.
Take control of your health with Craft Body Scan’s early detection services. Our locations across Tulsa, Texas, Florida, North Carolina, Tennessee, and Ohio provide accessible, affordable CT screening that identifies health concerns before they become serious. Board-certified radiologists review every scan, and our team helps you understand findings and next steps. Schedule your scan today and discover the power of early detection without the need for contrast agents.







